FERAHEME delivers clinical efficacy in just 1 gram1,2
In the largest IV iron head-to-head, double-blind, non-inferiority trial of ~2000 patients, 1 gram of FERAHEME vs 1.5 grams of Injectafer® demonstrated the following2:
body > div.bg-circles > div > div > main > div.row.main-page-row.theme_02 > div.col-lg-10.pages-content-block.-rellax > div.row.trial-comparison-items > div.trial-comparison-item.col-md.col-section_09 { display: none;}
FERAHEME
Injectafer®
Non-inferiority with respect to the composite endpoint of HSR and hypotension1,2
FERAHEME
Injectafer®
Significantly lower rate of severe hypophosphatemia1-3
FERAHEME
Injectafer®
The largest IV iron head-to-head, double-blind trial of ~2000 patients focused on incidence of hypersensitivity2
Incidence of moderate to severe HSR (including anaphylaxis) or moderate to severe hypotension2
-0.1%DIFFERENCE
(95% Cl, -0.91 to 0.70)
Non-inferiority
margin: 2.64%
P<0.0001
FERAHEME was non-inferior to Injectafer®2
| FERAHEME (n=997) Patients (%) | Injectafer® (n=1000) Patients (%) | |
|---|---|---|
| Primary endpoint: Composite of the following: |
6 (0.6%) | 7 (0.7%) |
| Moderate hypersensitivity reaction | 3 (0.3%) | 6 (0.6%) |
| Severe hypersensitivity reaction | 1 (0.1%) | 0 (0.0%) |
| Anaphylaxis | 0 (0.0%) | 0 (0.0%) |
| Moderate hypotension | 2 (0.2%) | 1 (0.1%) |
| Severe hypotension | 0 (0.0%) | 0 (0.0%) |
Please see Important Safety Information, including Boxed Warning.
FERAHEME does not require pretreatment
or a test dose prior to use
Moderate HSR was defined as requiring minimal medical intervention or corrective treatment; severe HSR was characterized as requiring medical intervention or corrective treatment, with possible hospitalization. Anaphylaxis was defined as a sudden, severe, potentially fatal systemic allergic reaction as operationally defined using the Sampson criteria. Hypotension was defined as a >30% decrease from baseline in systolic blood pressure or a decrease of >20 mm Hg in systolic blood pressure with symptoms (dizziness/lightheadedness, fatigue, syncope, lack of consciousness, blurred vision).2
CI=confidence interval; Hgb=hemoglobin; HSR=hypersensitivity reaction; IDA=iron deficiency anemia.
In the same study, FERAHEME had a significantly lower rate of severe hypophosphatemia vs Injectafer® (ferric carboxymaltose)1-3
Incidence of severe hypophosphatemia (defined by blood phosphorus of <0.6 mmol/L or <2.0 mg/dL at Week 2)1-3*
Patients were
91× MORE LIKELY
to experience severe
hypophosphatemia
with Injectafer®
(95% Cl, -41.5 to -35.2)
*An endpoint of severe hypophosphatemia was prespecified as serum phosphate level of <0.6 mmol/L or <2.0 mg/dL, which is defined as Grade 3 severity (severe or medically significant but not immediately life threatening; hospitalization; or prolongation of hospitalization indicated; disabling) by the Common Terminology Criteria for Adverse Events (CTCAE).3
FERAHEME demonstrated normal phosphate levels throughout the study period vs Injectafer® (ferric carboxymaltose)2,3
Serum phosphate levels2,3
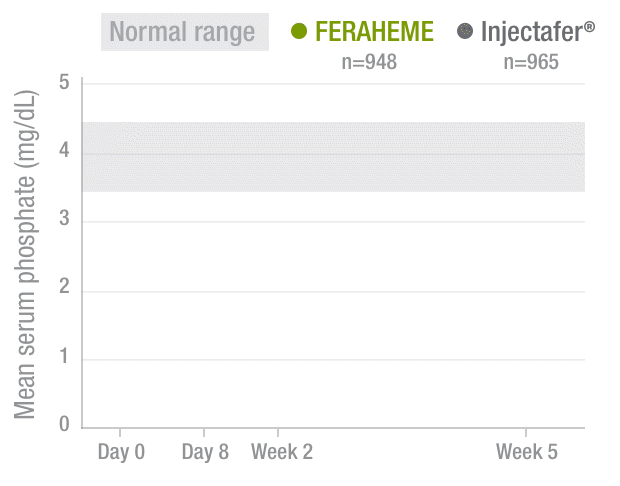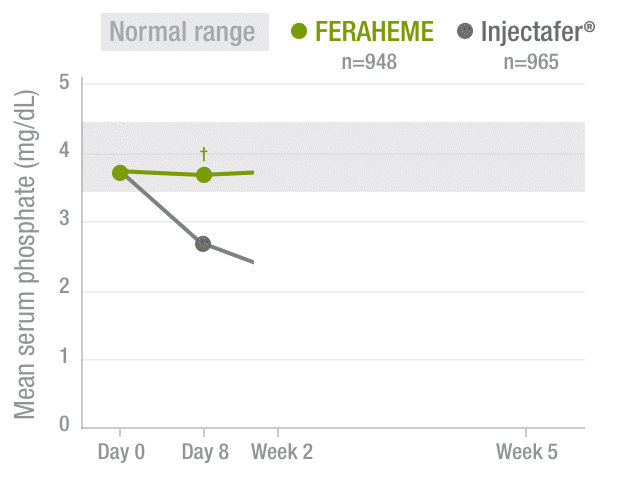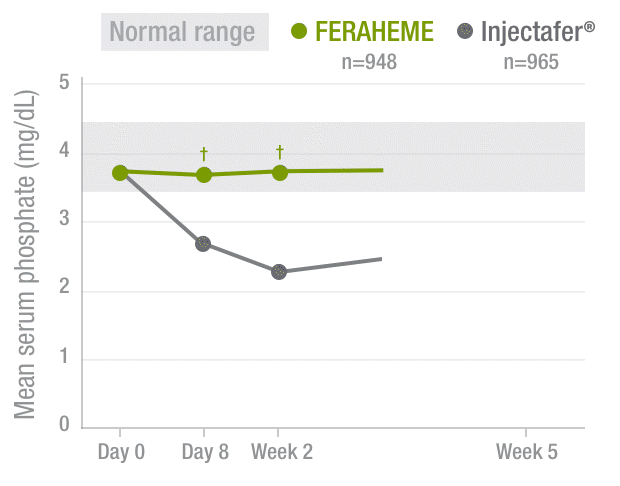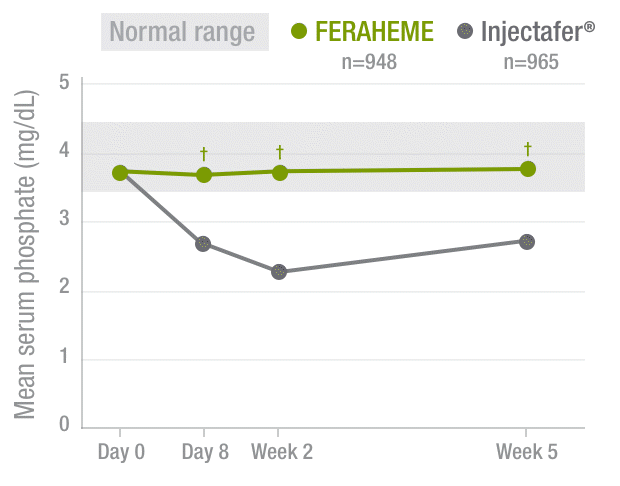
40%DECREASE
in mean serum
phosphate by Week 2
among Injectafer®–
treated patients vs
no change with
FERAHEME-treated
patients
†P<0.0001.
After 5 weeks, severe hypophosphatemia‡
was not transient as it persisted in 29.1% of Injectafer®-treated
patients compared with 0% of those treated with FERAHEME4
‡Defined by blood phosphorus of <2.0 mg/dL at Week 5.4
One gram of FERAHEME was non-inferior to 1.5 grams of Injectafer® (ferric carboxymaltose) with respect to Hgb rise at Week 51,2
Mean change in Hgb (g/dL) per full therapeutic dose1,2
-0.24g/dLDIFFERENCE*
(95% CI, -0.35 to -0.13)
Non-inferiority
margin: -0.5 g/dL
*Adjusted for difference in baseline Hgb.
Mean change in Hgb (g/dL) per gram of iron administered1,2
0.26 g/dLDIFFERENCE
(95% Cl = 0.17 to 0.36)
Non-inferiority
margin: -0.5 g/dL
Just 1 gram of FERAHEME demonstrated normal levels of mean ferritin and TSAT at Week 5 vs 1.5 grams of Injectafer® (ferric carboxymaltose)2,5,6
| FERAHEME (1.02 g) |
Injectafer® (1.5 g) |
Normal Range | |
|---|---|---|---|
| Ferritin levels Mean (SD) |
227.5 ng/mL (193.9 ng/mL) |
397.8 ng/mL (301.2 ng/mL) |
40-300 ng/mL(Male) 20-200 ng/mL(Female) |
| TSAT levels Mean (SD) |
25.9% (11.6%) |
28.6% (11.5%) |
20%-50% |
1 GRAM is the most common indicated dose of IV iron1,7,8
Select Important Safety Information: Excessive therapy with parenteral iron can lead to excess storage of iron with the possibility of iatrogenic hemosiderosis. Regularly monitor the hematologic response during parenteral iron therapy. Do not administer FERAHEME to patients with iron overload.
SD=standard deviation; TSAT=transferrin saturation.
FERAHEME demonstrated non-inferior efficacy to Venofer® in non-CKD and CKD patients.1
LEARN MORE

